Prof. Dr. med. Albert Bühlmann, University of Zürich
Published 1961 by J.R.Geigy S.A Switzerland «Der Weg in die Tiefe» in five bulletins
Respiratory Physiological Aspects of Diving
The medical-physiological difficulties of diving, especially deep diving, are closely related to respiration. The first to be mentioned is hyperoxia. Atmospheric air contains about 21% oxygen, giving an oxygen pressure in inspiratory air of about 140 Torr (mm Hg) at normal altitudes. In alveolar air the oxygen pressure is still 95, and in arterial blood about 90 Torr. This pressure is sufficient to saturate 95-97% of the hemoglobin of the red blood cells with oxygen.
Therefore, in normal lungs, higher oxygen pressures do not allow much higher oxygen saturation of the hemoglobin. The oxygen pressure of mixed venous blood is around 40 torr at rest, and less than 20 torr in the coronary veins. This means that the oxygen-carrying enzymes in our tissues are adapted to oxygen tensions on the order of 90-100 Torr on the arterial side and 20-40 Torr on the venous side. If pure oxygen is allowed to breathe, the oxygen pressure in the arterial blood rises to about 650 Torr, the hemoglobin becomes 100% saturated with oxygen, and about 7-8 times more oxygen is found physically dissolved in the plasma than when breathing air. While such conditions are well tolerated for many hours.
However, weeks of use can result in tissue damage, especially to the lungs and nervous system, as evidenced by the blindness of premature infants exposed to a pure oxygen atmosphere for weeks in incubators. The use of pure oxygen has been rightly frowned upon since this experience.
Paul Bert, a French respiratory physiologist, showed more than 50 years ago that in animal experiments pure oxygen at 3 atm can lead to death within a few hours.
3 atm of pure oxygen corresponds to an oxygen pressure in the alveoli and in the arterial blood of about 2300 Torr. Such flooding with oxygen results in inhibition of oxygen-carrying enzymes, and the animals die with the appearance of extensor spasms. Although these conditions have been known for a long time, diving equipment based on pure oxygen has always been built because it allows a closed breathing circuit, which provides a large reserve of time. In a closed circuit, the oxygen in the exhaled air is not lost, but returns to the system where the carbonic acid is chemically absorbed.
Compared to the open system, in which the expiratory air is directed into the water, it is theoretically possible to dive about 25 times longer with the same cylinder contents in the closed system; however, because of the limited capacity of the carbonic acid absorbers, this enormous time advantage cannot be fully exploited. With the closed system, only a few gas bubbles enter the water.
The diver’s path and location are therefore difficult to determine because of the almost complete absence of bubbles at the surface, which is of course a great advantage for military purposes, frogmen, human-guided torpedoes, etc. At a depth of 20 meters, however, such a diving apparatus already results in an oxygen pressure of 3 atm, which must lead to serious damage after a short time.
Deep diving with diving apparatus based purely on oxygen is therefore out of the question. Because of numerous accidents with such equipment, diving with 100% oxygen is forbidden in many countries today. However, the many accidents are not all due to oxygen poisoning; carbonic acid accumulation in the circuit with exhausted absorbers may also have been the cause of many accidents.
Compressed air contains 21% oxygen.
At a depth of 40 meters, at a total pressure of 5 atm, the oxygen pressure is already the same as at 100% oxygen at the surface, which, as mentioned, is well tolerated for several hours, but is no longer completely harmless. At a depth of 140 meters, at a total pressure of 15 atm, 21% oxygen already means an 02 pressure of 3.1 atm, i.e. about 2400 Torr, which is already the lethal oxygen pressure in animal experiments. Therefore, the oxygen content of the breathing mixture must be reduced for deep diving experiments. Of course, the time of exposure to pressure is also important. In case of a longer stay, a reduction of the oxygen concentration is even indicated for diving depths of 50 m. Symptoms such as increase in pulse rate and tachypnea are considered by some authors to be the beginning of oxygen toxicity with irritation of the adrenal sympathetic system.
Thus, it is quite consistent with physiological findings to reduce the oxygen concentration for diving in such a way that the partial oxygen pressure does not increase significantly above 500-600 Torr. Since a partial pressure of 130 to 140 Torr is sufficient for normal oxygen saturation of the hemoglobin, there is a considerable margin.
5% oxygen already allows normal oxygen saturation of the blood at a depth of 30 meters and only results in a partial oxygen pressure of 570 Torr at a depth of 140 meters, so that no damage is to be feared even after hours of exposure. A reduction in the oxygen concentration in the breathing mixture naturally means an increase in the nitrogen content.
Since many diving experts are of the opinion that nitrogen has a narcotic effect at high pressure and is also the cause of the deep intoxication, one has always resisted the reduction of the oxygen concentration and preferred to risk oxygen intoxication, although this was known and experimentally proven for a long time, while the «nitrogen narcosis» is a pure hypothesis that has not been proven by any experiment.
The reduction of the oxygen concentration in favor of nitrogen was the first respiratory physiologically justified measure in our deep diving experiments.
With this, 120 meters depth was reached without difficulty: no narcosis or deep intoxication occurred. However, it was clear to us that reducing the oxygen concentration merely as a preventive measure to avoid oxygen intoxication did not solve the problem of «deep intoxication» and the difficulties of deep diving.
On the other hand, new aspects emerged in the study of breathing mechanics, i.e. the determination of breathing resistances at overpressure. For a given oxygen uptake and carbon dioxide release, the diver must always deliver the same volume of ventilation as at the surface, regardless of pressure and depth, for example 10 liters per minute at rest or 30 liters per minute for moderately heavy work, swimming, etc. As the pressure increases, the gases become denser, the number of molecules per unit volume increases; the specific gravity of the gases increases in proportion to the compression. At 90 meters depth, at 10 atm total pressure, the same ventilation volume of, for example, 10 liters per minute, contains 10 times more molecules than at the surface, and is therefore also about 10 times heavier. The caliber of the airways remains constant, as does the ventilation volume. The viscosity of a gas, its viscosity, is independent of the pressure. This means that the flow resistance in a pipe system with laminar flow remains constant regardless of the pressure and thus the weight of the gas.
Now, however, air flow in human airways is partly turbulent, and the proportion of turbulent flow, moreover, increases with increasing current, i.e., with increasing ventilation volume. However, once turbulence is in play, flow resistance increases with gas density (the specific gravity of the gas).
As a first approximation, for a given ventilation volume, bronchial flow resistances at 10 atm total pressure (90 meters depth) for the same gas, for example, compressed air, are 10 times higher than at the surface at 1 atm. This means that at a depth of 90 meters, the respiratory muscles have to do about 10 times more work to overcome bronchial flow resistance for a given respiratory minute volume. However, the capacity of the respiratory musculature is limited. Breathing at a depth of 90 meters, even with a relatively small respiratory minute volume, becomes as difficult as in the case of bronchial obstruction, for example, an attack of bronchial asthma.
The diagram shows how, when compressed air is used, the maximum ventilation capability, for example 120 liters per minute at the surface, decreases rapidly with increasing depth. Maximum ventilation with corresponding effort of the entire respiratory musculature is only possible for a few seconds anyway.
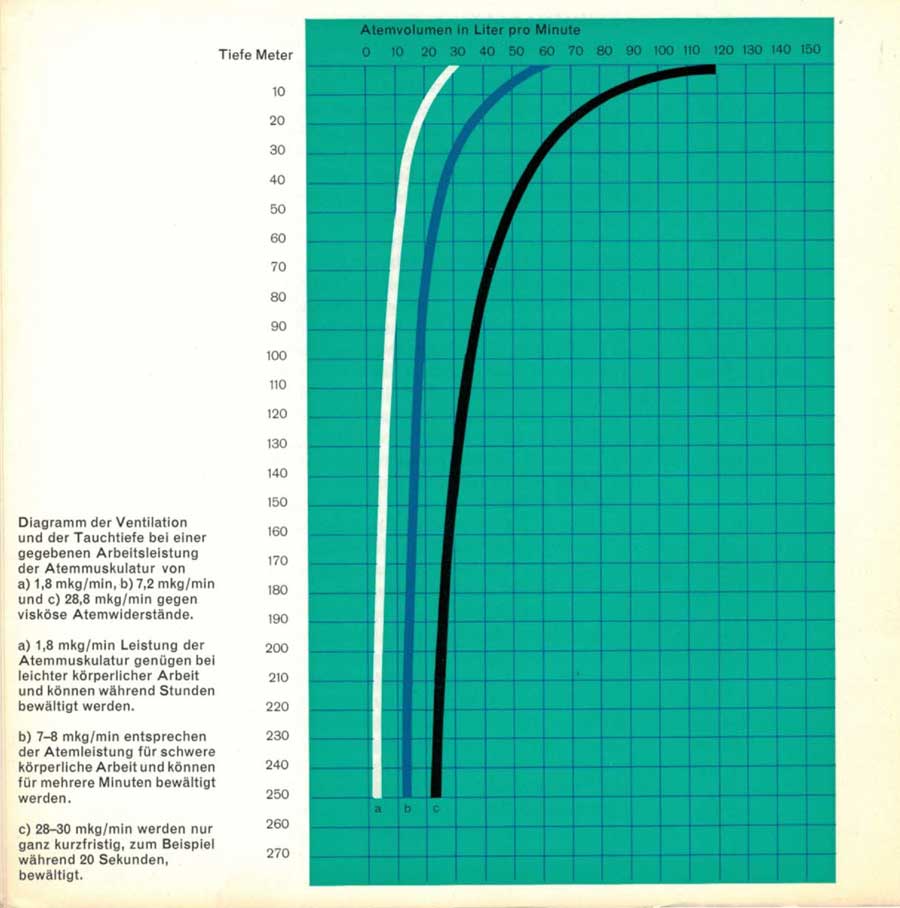
a) 1.8 mkg/min
b)7.2 mkg/min
c) 28.8 mkg/min
against viscous breathing resistances.
a) 1.8 mkg/min of respiratory muscle power is sufficient for light physical work and can be performed for hours.
b) 7.2 mkg/min corresponds to the breathing capacity for heavy physical work and can be performed for several minutes.
c) 28.8 mkg/min can only be performed for a very short time, e.g. 20 seconds.
Our respiratory mechanics studies show that subjects who are not particularly athletically trained can manage a ventilation volume of 60-70 liters per minute for several minutes during heavy physical work. With the same effort of the respiratory muscles, a ventilation volume of only about 20 liters per minute is possible with compressed air at a depth of 80 meters, which is only sufficient for an adequate gas exchange during very light work. Unless the diver is at rest and requires a ventilation volume of more than 15-20 liters per minute, at diving depths of 80 meters and more he will very soon come into conflict between the need for ventilation and too great a strain on the respiratory muscles. As in a severe asthma attack at the surface, hypoventilation develops in this situation (global insufficiency according to Rossier).
Hypoventilation, however, absolute or relative, means carbonic acid retention with all its consequences, acidosis, increase of blood pressure in the body and pulmonary circulation, increase of cerebral blood flow and increase of intracranial pressure. In diving, the tendency to reduce the ventilation volume, i.e., to hypoventilate, when the respiratory muscles are under too much strain is further supported by the fact that, in contrast to surface diving, arterial hypoxemia does not occur despite hypoventilation because the partial oxygen pressure is greatly increased. Thus, the influence of hypoxemia-sensitive receptors in the glomus caroticum on respiratory regulation is absent. The combination of hyperoxia and maximum load on the respiratory muscles always favors hypoventilation, as clinical experience with oxygen therapy, which is usually contraindicated in obstructive pulmonary diseases, bronchial asthma, etc., shows. Patients do calm down, become less cyanotic, eventually fall asleep, and may enter carbonic anesthesia, which is subjectively perceived as pleasant.
What could be more obvious than to assume a prenarcotic stage of carbonic acid retention in the so-called deep intoxication?
This concept would explain the phenomena described by divers, the occurrence at a certain, individually varying depth, the rapid disappearance when returning to shallower depths, where ventilation can be increased immediately due to the smaller strain on the respiratory muscles and the retinated carbonic acid can be exhaled, as well as the absence of the deep intoxication even when staying for hours at depths of 30-40 meters.
We have come to these conclusions on the basis of respiratory mechanics measurements and theoretical considerations. American as well as German authors have been able to prove in the hyperbaric chamber that the maximum possible ventilation, the breathing limit, decreases according to the overpressure, as it is to be expected according to the diagram. Both groups of investigators also found an increase in arterial and alveolar carbonic acid tension, respectively, as evidence of hypoventilation, but without being able to give an explanation, because the respiratory mechanics aspects about the influence of the overpressure on the bronchial flow resistances were not taken into account.
Deep diving with compressed air is very difficult, especially when physical work is to be performed, because of the great strain on the respiratory muscles and the danger of hypoventilation with carbonic acid retention.
From the respiratory physiology point of view, there are two solutions to this problem. The respiratory muscles can be relieved by having the respiratory work mainly performed by an apparatus with its own power source that adapts to spontaneous breathing, as we do in the clinic for artificial respiration of patients with respiratory paralysis. The construction of such breathing aids suitable for diving is only in its infancy.
The other possibility is to replace the heavy nitrogen, which is not involved in gas exchange at all, with a specifically lighter gas that is also inactive. Helium, for example, is about 7 times lighter than nitrogen, which results in a considerable rightward shift of the curves shown in the diagram, so that ventilation volumes of 30 liters per minute and more are possible even at greater diving depths. With the even lighter hydrogen, which is also much cheaper, a further gain would be possible. In terms of breathing difficulty, depths of 500-600 meters can be reached without difficulty with such gas mixtures.
The Americans have been using helium routinely for about 30 years. The proposal to use helium instead of nitrogen for diving was made before the First World War. However, the respiratory physiological problems involved were only partially studied, and American experts still believe that nitrogen has a narcotic effect at high pressure and should therefore be replaced by helium. In spite of their great practical experience with helium-oxygen mixtures, Americans rarely dive deeper than 60-70 meters.
A Swedish diver, Zetterström, used a hydrogen-nitrogen-oxygen mixture in a deep dive attempt in 1945, but lost his life in the attempt, which did not encourage further experimentation with hydrogen-air mixtures.
With the introduction of helium or hydrogen, the problem of dyspnea could be solved, but new and unexpected difficulties arose in connection with decompression, the safe resurfacing, which outweighed the practical advantages of these light gases, so that deep diving nevertheless remained limited at about 120 m. The difficulties of decompression will be discussed in a special bulletin.
Finally, a rare accident possibility should be pointed out. If the intraalveolar pressure is greatly increased with respect to the external pressure, for example, during a strong coughing thrust, lung rupture, penetration of air into the vessels and consequent air embolism, mediastinal emphysema, and spontaneous pneumothorax may occur. This risk is particularly present when, due to partial bronchial stenosis, a valve mechanism is created that obstructs air outflow, a phenomenon known as «air trapping.» During diving, the valves of the breathing apparatus automatically ensure that the pressure of the breathing gas corresponds to the water pressure at the time, so that the intrathoracic pressure changes generated by the respiratory muscles are sufficient, on the order of a few Torricelli (mm Hg), for inhalation and exhalation. The absolute intrathoracic pressure must be equal to the water pressure so that the thorax is not compressed during diving and does not burst during resurfacing.
Now, in the case of poorly functioning breathing valves, as well as in the case of uneven flow resistance in the airways, especially in the case of bronchitis, it is quite possible that during ascent, similar to coughing in poorly ventilated parts of the lungs, a positive alveolar pressure in the order of 70-80 Torr compared to the external pressure may occur, leading to rupture of alveolar septa. Such accidents involving air emboli have been described repeatedly, but are likely to be rare overall.
The breathing valves available today, whose development was significantly influenced by Cousteau, guarantee a very fast and precise regulation of the pressure of the breathing gases according to the respective water pressure, i.e. the depth. Nevertheless, in the medical care of divers, attention must be paid to the presence of bronchitis. Bronchial asthma, chronic asthmoid bronchitis, bronchiectasis and obstructive emphysema are synonymous with inability to dive.





