Prof. Dr. med. Albert Bühlmann, University of Zürich
Published 1961 by J.R.Geigy S.A Switzerland «Der Weg in die Tiefe» in five bulletins
Decompression, the safe resurfacing
The problem of greatest practical importance remains for diving, especially deep diving, decompression, the safe resurfacing. Most diving accidents and the so-called caisson disease are due to insufficient decompression. The problem is as old as diving and has always concerned divers physiologists and medical advisors. What is it all about?
During diving, the whole organism is under increased pressure according to the diving depth. In order for the thorax not to be compressed, the gas breathed, compressed air, oxygen or any mixture, must have the same pressure as the water column, otherwise spontaneous breathing would not be possible at all. In the lungs, all breathed gases are in contact with the circulating blood through an alveolar-capillary surface of about 150 m2 . All gases are physically dissolved according to their partial pressure in the blood as in any other liquid.
When breathing compressed air, the blood contains 10 times more nitrogen after full saturation at 90 meters depth, i.e. at 10 atm, than at the surface.
At constant pressure and temperature, the absolute amount depends only on the specific solubility factor in the environment concerned. Since all circulating blood passes through the lungs at least once per minute, the additional gas uptake into the blood occurs very rapidly. The blood now distributes the dissolved gases throughout the organism. The various organs and tissues also absorb additional gas according to the size of their blood flow. The venous blood then contains slightly less gas and saturates again during the next lung passage according to the gas pressure in the alveoli. Well perfused organs, such as the brain and kidneys, reach full saturation after a few minutes according to the current pressure. Poorly perfused tissues, such as the muscles, fatty tissue or joints, take longer to reach full saturation, in some cases up to two hours. The total amount of additional dissolved gases in the organism depends only on time, assuming constant temperature and pressure until all tissues are fully saturated. For example, during a one-hour stay at a depth of 30 meters, considerably more nitrogen is additionally dissolved when breathing compressed air than during a stay of 5 minutes at a depth of 100 meters.
This quantitative consideration explains the very different decompression times, which depend not only on the depth, but above all on the duration of exposure to overpressure. When the diver ascends again, the pressure decreases. At this moment, blood and tissues represent a solution supersaturated with gases, which must release these gases. If the critical supersaturation is exceeded, the excess of physically dissolved gases in blood and tissues changes to the gaseous phase with a corresponding increase in volume. Gas bubbles are formed, just as the carbonic acid under excess pressure in a mineral water bottle escapes in gaseous form when the bottle is opened. Such gas bubble formation in the blood is life-threatening because of the gas embolism in the coronary vessels and in the brain.
Bubble formation in the brain and in the nervous tissue itself naturally leads to severe neurological deficits as a result of the mechanical damage to the tissue.
It does not matter what gas the bubble is made of, because it acts as a mechanical obstacle in the bloodstream or as a displacing, space-occupying foreign body in the tissue. The only exception is the oxygen bubble, because this gas, which is involved in metabolism, can be immediately absorbed and chemically bound in any tissue. Gas bubble formations in fatty tissue, muscles and joints are not life-threatening, but they are painful; here they act like other foreign bodies and can eventually lead to arthrosis (see figure).
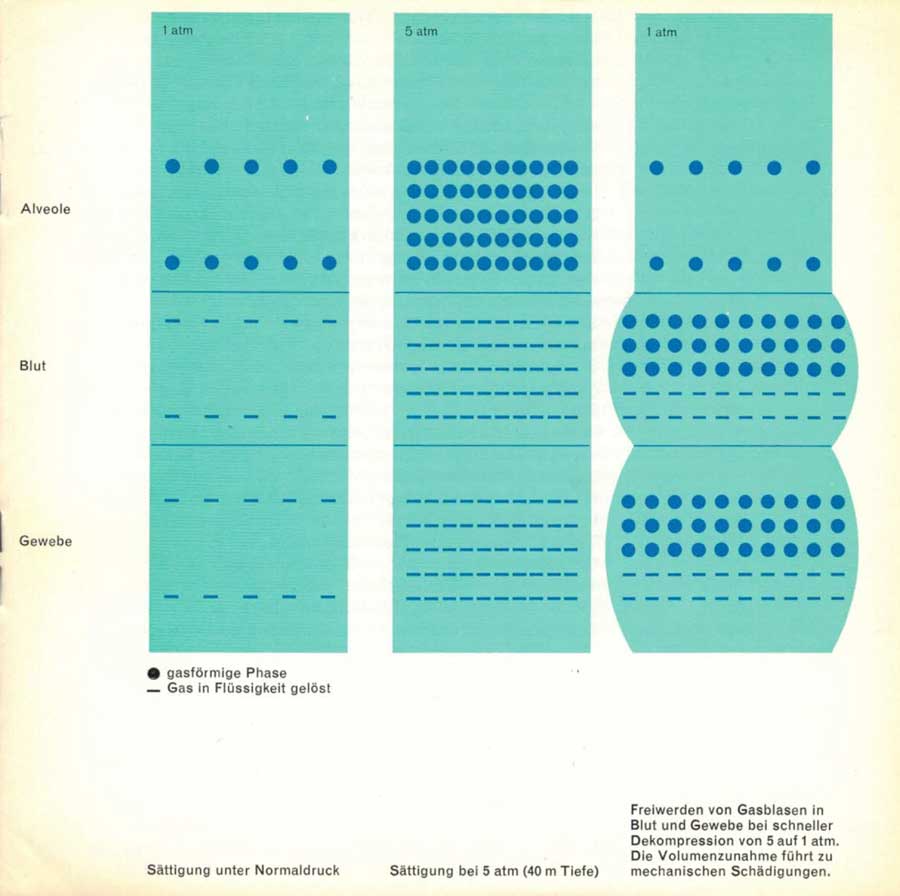
2nd row: Saturation at 5 atm (40 metres depth)
3rd row: Release of gas bubbles in blood and tissues during rapid decompression from 5 to 1 atm. The increase in volume leads to physical damage.
The delayed resurfacing has the purpose of releasing the additional gases dissolved under overpressure from the various tissues through the blood to the lungs and thus to the outside air in such a way that no gas bubbles can form either in the tissues or in the blood. The additionally dissolved gases must leave the organism in the opposite direction by the same route by which they entered the organism. Since the blood itself has very good contact per unit of time with an optimal surface such as the lungs, gas bubble formation in the blood is possible only in the case of very brusque decompression, in actual accidents. The situation is different with the poorly perfused tissues, especially fat, muscles and joints. Here, even with slow decompression, which prevents any bubble formation in the blood, bubbles can still appear after hours.
The caisson disease of many professional divers is due to mechanical damage to the tissues as a result of such late bubble formation, especially in the joints. Blood and tissues, because of their composition of water, cells, proteins and electrolytes, allow a solution supersaturated with gas compared to pure water. Gas bubble formation occurs after full saturation in accordance with the overpressure, not as in water even at a low pressure, but only at a greater reduction in pressure. Without this biological fact, diving, like flying, would be practically impossible without a pressurized cabin.
Haldane arrived purely empirically at a critical supersaturation factor of 2:1. He observed that after a dive of any length, as well as after repeated dives at a depth of 10 meters, one can return to the surface immediately in each case without any damage occurring. It is therefore possible to halve the pressure within a short time without gas bubbles forming in the blood or tissues, although at that moment, at full saturation, twice as much gas is dissolved as corresponds to the current pressure. This creates a considerable pressure gradient between the gases in the blood and in the lungs, which is what makes the release to the alveolar air possible in the first place. The critical supersaturation factor of 2:1 applies to air containing 21% oxygen. Since this gas, which is involved in metabolism, does not form bubbles, the supersaturation factor, in terms of nitrogen, is reduced by 21% and is 1.8. It can be assumed that different tissues have somewhat different specific supersaturation factors, which has not yet been sufficiently studied in detail.
Of greatest practical importance is that the critical supersaturation is independent of the absolute pressure.
Just as one can immediately ascend from a depth of 10 meters without danger, one may also, for example, quickly rising up from a depth of 90 meters to 40 meters, because this also corresponds only to a halving of the pressure from 10 to 5 atm. Then a stop must be activated, during which the excess gases can be released from the tissues via the blood through the lungs. If the gas saturation again corresponds to the current pressure, the next pressure reduction can take place. As the pressure differences become smaller and smaller, the release of gas takes more time than at the beginning of the ascent. Thus, the decompression process follows an asymptotic curve to normal pressure at the surface.
After a dive, the diver is bound to a «minimum depth» that ideally just corresponds to the critical supersaturation of the tissue that is still most saturated at the moment. Then no gas bubbles are released anywhere; on the other hand, a maximum gas release per time unit is guaranteed. Conversely, there is also a «maximum depth» at which the pressure is still so high that no tissue with its gas content is close to the critical supersaturation and thus no or only a small release of gases can occur. The efficiency of the «decompression ceremony» is the better, the closer it follows the curve of critical supersaturation.
Decompression tables of all kinds indicate the depths at which one should stay for certain times after a dive. It often happens that a diver – thinking he is doing particularly well – does not go below the indicated depths, but exceeds them. Because in this way only the gas release is slower, only the efficiency of the decompression is worsened. According to the table data, there is always a tendency to perform decompression in abrupt stages. A slowly controlled ascent, ascending to the next stage during the time allowed for each stage, gives the best efficiency.
Today, decompression is of practical importance not only for diving but also for flying. Modern jet fighters reach 5500 meters in a few seconds, where the air pressure is only 380 Torr, half the pressure on the ground. In this case, too, the critical supersaturation for nitrogen is reached in blood and tissues. A rapid ascent to higher altitudes would therefore result in gas embolism if the aviator were not equipped with a pressure suit or the aircraft were not equipped with a pressurized cabin. If the pressurized cabin becomes defective in a high-altitude aircraft, bubble formation also occurs with the brusque drop in pressure. The pilot must therefore immediately go lower, i.e. into an area of higher air pressure. Below 5500 meters, there is no longer any danger of bubble formation.
The decompression tables in use today for diving are all based on the studies of Haldane, but he only considered decompression for the use of nitrogen-oxygen mixtures and compressed air up to 90 meters depth.
During World War II, the English and Americans in particular published modified tables based on new investigations and calculation methods, some of which resulted in shortened decompression times for certain diving depths. The American Dwyer tried for the first time in 1955 to work out the whole problem mathematically with a calculating machine. The decompression times he calculated were then modified experimentally. They certainly represent progress compared to the old tables, although they did not bring any new insights in principle. These tables also provide many hours for decompression at greater diving depths, such as 100 meters, for longer stays.
With the introduction of helium for deep diving, new problems arose for decompression as well. Initially, it was believed that a shortening of decompression time would result. Decompression accidents involving helium divers then showed that just the opposite was true. Helium is not only lighter than nitrogen and can therefore replace it in deep diving to relieve the respiratory muscles (see Bulletin 3), it also diffuses more quickly into the blood and tissues. Thus, tissue saturation is achieved in a shorter time than with nitrogen. Decompression must be performed as if more or less complete saturation of the tissues had been achieved, even if the descent is only for a short time. With helium, the problem of dyspnea and carbonic acid narcosis during deep diving could be solved, but one had to accept a sometimes considerable prolongation of decompression times. In addition, if decompression takes place exclusively in the water, this can lead to the diver freezing to death if no heating is installed in his suit. The thermal insulation provided by the air jacket in the diving suit, although very good, is not complete and cannot prevent cooling even at shallow depths if the diver spends many hours in cold water.
These difficulties explain why deep diving has been limited to 80-100 meters and why the «Andrea Doria» is still at the bottom of the sea.
Can the prohibitive long decompression times of deep diving be drastically shortened without endangering the diver, so that ship salvage, oil drilling, etc. at depths of 100 meters and more by free divers become possible?
Physical and biological laws cannot be circumvented, but their very rigidity also offers some positive possibilities. A long known and occasionally used way to shorten the decompression time is to breathe pure oxygen for a longer time, for example 2-3 hours, before the dive. Blood and tissues are saturated with nitrogen according to the nitrogen content of atmospheric air of 79% – which at normal atmospheric pressure gives a partial nitrogen pressure in inspiratory air of 570 Torr. In one liter of whole blood there is already normally 9.8 ml of nitrogen and in the whole organism 700 ml in terms of size. If pure oxygen is now breathed, the blood releases its nitrogen to the alveoli, because a nitrogen pressure gradient is created between the blood and the nitrogen-free alveolar gases. If the nitrogen pressure in the blood drops, the tissues also release their physically dissolved nitrogen to the blood. In this way, all the nitrogen dissolved in the body can be «washed out» by oxygen respiration. If compressed air is then breathed during diving, nitrogen saturation starts at zero, so to speak. It takes some time before even as much nitrogen is dissolved again as would already be present in an atmosphere. It is obvious that this time reserve gained in this way is limited and can only have its full effect on short-term dives lasting less than an hour.
For example, if the diver breathes pure oxygen for one hour before the dive, he can stay at a depth of 50 meters for 21 minutes and then return to the surface in 5 minutes, i.e. very quickly, without risking major decompression damage; without oxygen breathing before the dive, the maximum time spent at this depth without subsequent decompression is limited to 14 minutes. The time gain for this depth is 7 minutes. It becomes smaller with increasing depth, and at a depth of 100 meters it amounts to only 3 minutes for a stay of 7 minutes. Thus, the effect becomes smaller the deeper you go and the longer you stay.
Oxygen breathing before the dive therefore only allows a significant reduction of the decompression time for very short descents, as we have done so far. It was explained above that the correct decompression procedure must follow an asymptotic curve in time. The rate of ascent becomes slower and slower, and the time required for the last stages becomes longer and longer, the closer the diver gets to the surface, because the pressure gradient of nitrogen between tissues, blood and alveolar air becomes smaller and smaller in absolute terms, which results in slower nitrogen elimination. If the compressed air for the last stages between 10 meters depth and the surface is replaced by oxygen, the pressure gradient for the nitrogen is increased again and a faster excretion of the excess nitrogen is achieved, which enables a further shortening of the decompression time. Unfortunately, because of the toxic effect of oxygen at overpressure (see Bulletin 3), this method of decompression shortening can only be considered for the last stages near the surface, where the oxygen pressure is less than 2 atm. On the other hand, this application is also effective for decompression shortening during longer dives.
However, since the oxygen may be toxic even at low overpressure in case of repetition, this option should be used with caution for dives repeated at short intervals. During our successful deep diving tests at 155 meters depth in Lake Maggiore and 250 meters in the overpressure chamber of the French Navy in Toulon, we managed with very short decompression times of less than one hour.
The next tests will demonstrate that it is possible to stay and work at such depths for some time – which would have practical significance – and still manage with economically acceptable decompression times.
The principle of our new decompression method will not be discussed in detail here. The application was only possible after we had collected all known physical gas factors and biological constants, such as gas solubility in various media, blood flow to the organs, etc., and had mathematically considered all possibilities. However, the prerequisite for such calculations is considerable mathematical knowledge, and then the execution of a huge computational program is necessary, which can only be realized in a useful period of time with the use of an electronic calculating machine.
On the basis of 250,000 individual data obtained in this way, it was then possible to develop a new decompression method, which can be further improved with more precise knowledge of some other basic data to be determined experimentally. The whole dive with descent duration, length of stay, heaviness of work, swimming, etc. and finally the resurfacing must be precisely programmed beforehand and controlled afterwards from above, not by the diver himself. Unforeseen incidents and time shifts must be recognized during the dive and considered in terms of their importance to decompression. The diver is no longer the sole actor and star, but part of a team whose members must perform precisely circumscribed tasks. If deep diving acquires practical significance, this will only be possible with considerable technical effort and precise work on the part of all those involved, which puts the romantic aspects of diving somewhat in the background.





